Author: Medical Updates
- E-cigarettes, also known as vapes, are battery-operated devices that heat a liquid and produce an aerosol. An aerosol is a mix of small particles released in the air. Some people refer to the aerosol from an e-cigarette as “vapor.”
- A person using an e-cigarette inhales this aerosol into their lungs. Bystanders can also breathe in the aerosol when the person using the e-cigarette breathes the aerosol out.
- E-cigarettes typically contain nicotine, the addictive substance in cigarettes and other tobacco products. Some e-cigarettes can also be used to deliver cannabis and other drugs.
- E-cigarettes often come in bright colors. They are also available in flavors that appeal to young people, like fruit, candy, menthol, and mint.
- People often refer to using e-cigarettes as “vaping.”
The U.S. Food and Drug Administration (FDA) has authorized the marketing of four menthol-flavored e-cigarette products by NJOY LLC in the United States. This authorization, granted through the premarket tobacco product application (PMTA) pathway, applies to NJOY ACE Pod Menthol 2.4%, NJOY ACE Pod Menthol 5%, NJOY DAILY Menthol 4.5%, and NJOY DAILY EXTRA Menthol 6%. The ACE products are sealed, pre-filled, non-refillable pods used with the ACE device, and the DAILY products are disposable e-cigarettes with prefilled, non-refillable e-liquid reservoirs.
These authorizations are notable as they are the first non-tobacco flavored e-cigarette products authorized by the FDA. Each application is reviewed individually, and today’s actions apply only to these four products. Companies must receive a written marketing order from the FDA to legally market a new tobacco product in the U.S. This action allows these specific products to be marketed legally but does not mean they are safe or “FDA approved.” All tobacco products are harmful and potentially addictive, and those who do not use them should not start.
Brian King, Ph.D., M.P.H., director of the FDA’s Center for Tobacco Products, emphasized the responsibility of applicants to provide the necessary evidence for marketing authorization. He stated, “This action is further reinforcement that authorization of an e-cigarette product is possible when sufficient scientific evidence has been submitted to the agency to justify it.”
The FDA evaluates PMTAs based on a public health standard that considers the product’s risks and benefits to the population as a whole. After reviewing the company’s applications, the FDA determined sufficient evidence demonstrated that marketing these products would be appropriate for public health protection, as required by the 2009 Family Smoking Prevention and Tobacco Control Act. Specifically, the evidence showed that these menthol-flavored products benefit adults who smoke cigarettes, promoting complete switching compared to the company’s previously authorized tobacco-flavored products.
Matthew Farrelly, Ph.D., director of the Office of Science in the FDA’s Center for Tobacco Products, stated, “Based upon our rigorous scientific review, in this instance, the strength of evidence of benefits to adult smokers from completely switching to a less harmful product was sufficient to outweigh the risks to youth.”
Bottom Line:
- No tobacco products, including e-cigarettes, are safe.
- Most e-cigarettes contain nicotine, which is highly addictive and is a health danger for pregnant people, developing fetuses, and youth.
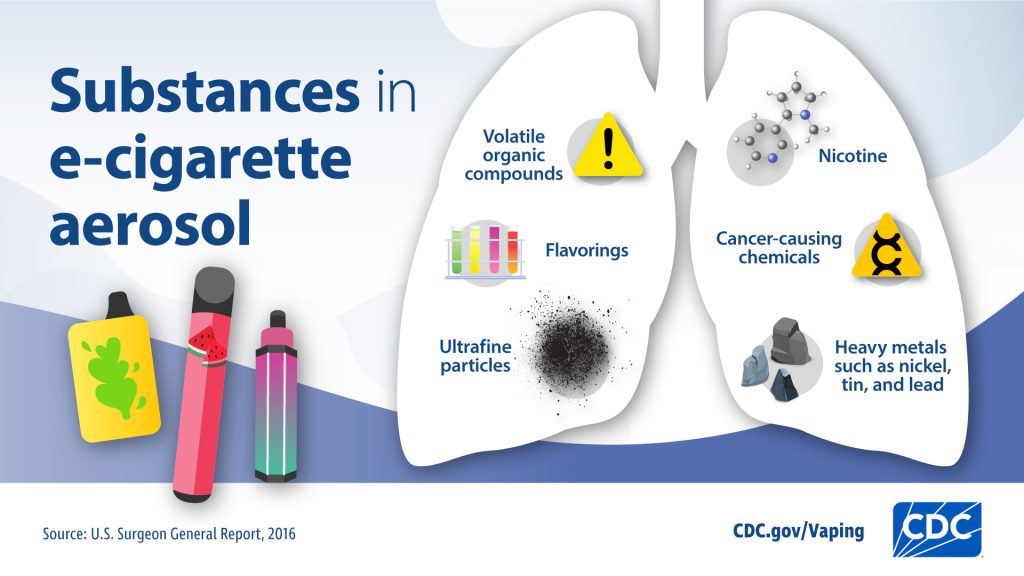
- Aerosol from e-cigarettes can also contain harmful and potentially harmful substances. These include cancer-causing chemicals and tiny particles that can be inhaled deep into lungs.
- E-cigarettes should not be used by youth, young adults, or people who are pregnant. E-cigarettes may have the potential to benefit adults who smoke and are not pregnant if used as a complete substitute for all smoked tobacco products.
- Scientists still have a lot to learn about the short- and long-term health effects of using e-cigarettes.
Source: FDA & CDC

